At a scale of 1 to 100 nanometers (nm) — one-billionth of a meter — nanoparticles are too small to be visible through a traditional microscope. But this extremely small scale makes them potential candidates for targeted drug delivery, capable of precisely pinpointing disease sources with increased efficiency and minimal side effects to surrounding tissues.
Courtesy of BIDMC
Tracking nanoparticles
Findings could help in developing treatment for pulmonary disease
Using a real-time imaging system, scientists have tracked a group of near-infrared fluorescent nanoparticles from the airspaces of the lungs into the body and out again, providing a description of the characteristics and behavior of the particles that could be used in developing therapeutic agents to treat pulmonary disease. The findings could also offer a greater understanding of the health effects of air pollution.
Led by investigators at Beth Israel Deaconess Medical Center (BIDMC) and the Harvard School of Public Health, the findings are described in the Nov. 7 advance online issue of the journal Nature Biotechnology.
At a scale of 1 to 100 nanometers (nm) — one-billionth of a meter — nanoparticles are too small to be visible through a traditional microscope. But this extremely small scale makes them potential candidates for targeted drug delivery, capable of precisely pinpointing disease sources with increased efficiency and minimal side effects to surrounding tissues.
“Nanoparticles hold promise as therapeutic agents for a number of diseases,” says co-senior author John V. Frangioni of the Division of Hematology/Oncology at BIDMC and associate professor of medicine and of radiology at Harvard Medical School (HMS), whose laboratory specializes in the development of imaging systems and of contrast agent development for molecular imaging. The anatomy of the lung, with its large surface area and minimal barriers limiting access to the body, makes it a good target for nanoparticle drug delivery.
“We have been interested in the fate of small particles after they deposit deep in the gas-exchange region of the lung,” says co-senior author Akira Tsuda, a research scientist in the Molecular and Integrative Physiological Sciences Program in the Department of Environmental Health at the Harvard School of Public Health. “Determining the physiochemical characteristics of inhaled nanoparticles on their ability to cross the [lungs’] alveolar epithelial surface is an important step in understanding the biological effects associated with exposure to these particles.”
Previous work by Frangioni and first author Hak Soo Choi, an instructor in medicine at HMS, had established the characteristics of nanoparticles that regulate clearance from the body. “To be of value clinically, nanoparticles must be able to either biodegrade into biologically inert compounds, or be efficiently cleared from the body,” says Choi, explaining that accumulation of nanoparticles can be toxic.
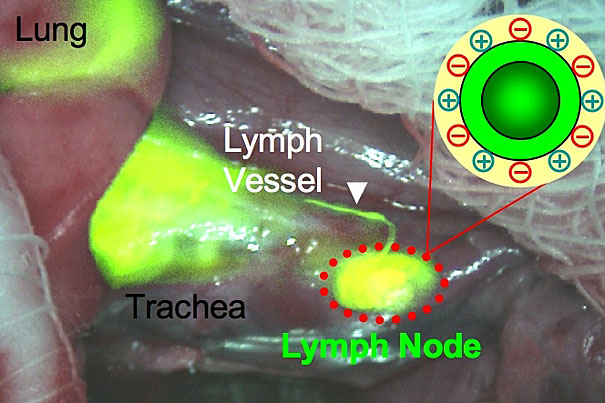
The aim of this new study was to determine the characteristics and parameters of inhaled nanoparticles that mediate their uptake into the body — from the external environment, across the alveolar lung surface and into the lymphatic system and blood stream and eventually to other organs. To do this, the scientists made use of the fluorescence-assisted resection and exploration (FLARE) imaging system, systematically varying the chemical composition, size, shape, and surface charge of a group of near-infrared fluorescent nanoparticles to compare the physiochemical properties of the various engineered particles. The investigators then tracked the movement of the varying nanoparticles in the lungs of rat models over a period of an hour, and also verified results using conventional radioactive tracers.
“The FLARE system enabled us to cut the number of experiments in half while performing direct comparisons of nanoparticles of different sizes, shapes, and rigidities,” says Frangioni, whose laboratory developed the FLARE system for use in image-guided cancer surgery as well as other applications.
The results established that nonpositively charged nanoparticles, smaller than 34 nm in diameter, appeared in the lung-draining lymph nodes within 30 minutes. They also found that nanoparticles smaller than 6 nm in diameter with “zwitterionic” characteristics (equal positive and negative charge) traveled to the draining lymph nodes within just a few minutes, subsequently being cleared by the kidneys into urine.
“These new findings can be applied to design and optimize particles for drug delivery by inhalation therapy,” notes Tsuda. “This research also guides us in the assessment of the health effects of various particulate pollutants, as the data suggest the importance of distinguishing specific subclasses of particles [based on surface chemistry and size] that can rapidly cross the alveolar epithelium and may disseminate in the body.”
Adds Frangioni, “This study complements our earlier work in which we defined the characteristics of nanoparticles that regulate efficient clearance from the body. With these new findings, which define the characteristics that regulate uptake into the body, we’ve now described a complete ‘cycle’ of nanoparticle trafficking — from the environment, through the lungs, into the body, then out of the kidneys in urine and back to the environment.”
The work was supported, in part, by grants from the National Institutes of Health.




