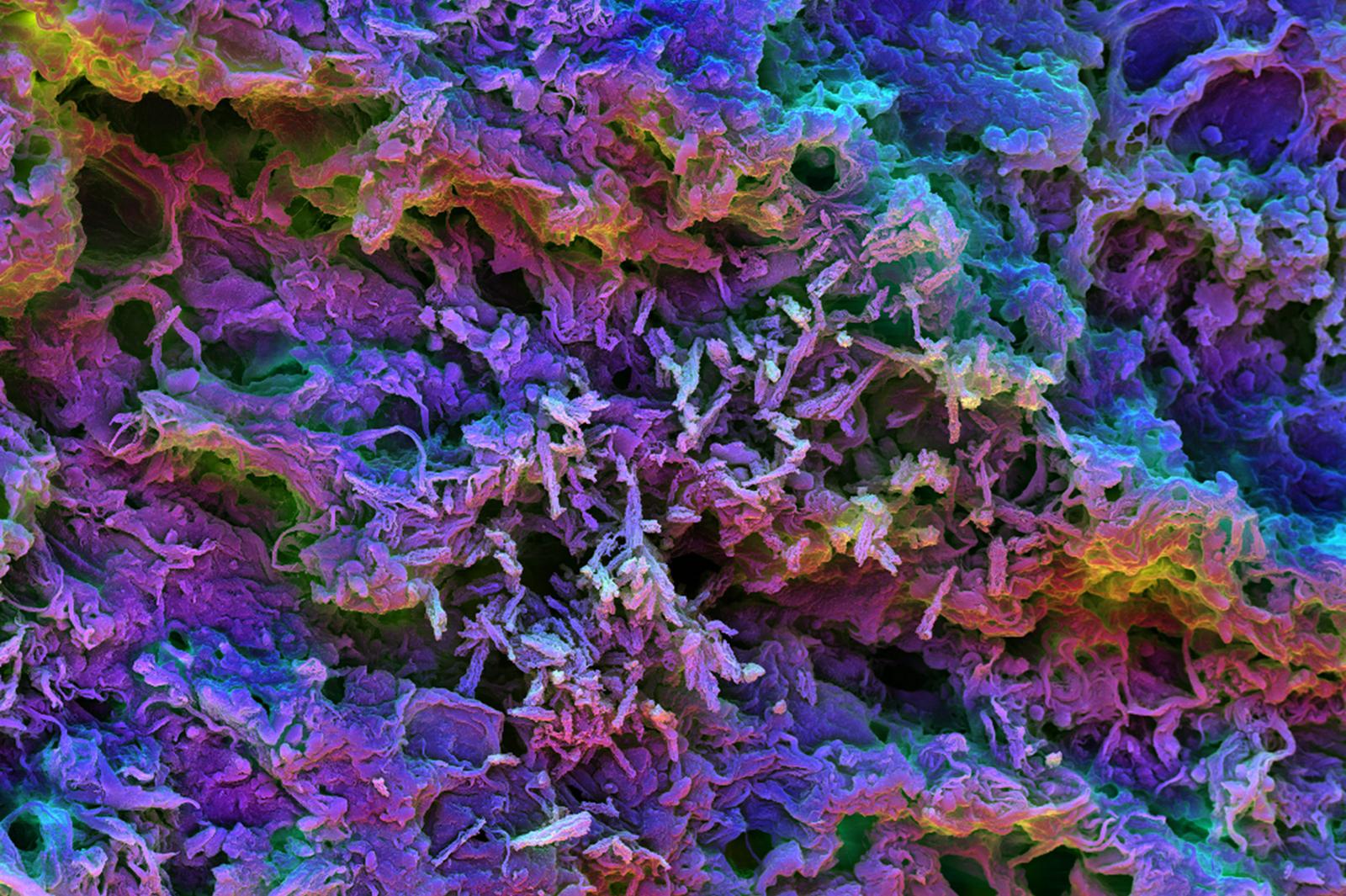
This scanning electron micrograph image shows the MSR-PEI scaffold presenting tumor-expressed peptides. After it’s injected under the skin of mice, the biomaterial fills with dendritic cells that can be seen here as small, round shapes interacting with the spiky scaffold structure.
Credit: Wyss Institute at Harvard University
Personal cancer vaccines show promise
Tumor-specific peptides could help eradicate tumors and prevent them from recurring
Immunotherapies are moving to the forefront of cancer treatment. Recent clinical trials have demonstrated that these approaches can be personalized to the unique mutations profile of each individual’s tumor, igniting new hope for many patients, according to a new Harvard study.
At the base of these developments are tumor-specific “neoantigens,” mutated peptides that tumor cells present on their surfaces. After taking up neoantigens, the immune system’s dendritic cells (DCs) can initiate strong T cell responses to attack the very cancer cells that express them, meaning they stimulate patients’ immune systems to destroy their own tumors. But despite that success, it has remained difficult to integrate different types of peptides into “cancer vaccines” that the immune system will accept.
The new study by a team of researchers at Harvard’s Wyss Institute for Biologically Inspired Engineering, the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), and the Dana-Farber Cancer Institute describes a vaccine approach that uses an injectable scaffold loaded with a selection of tumor-expressed peptides.
In a paper published in Nature Methods, Wyss Institute core faculty member David Mooney and his team explain how this strategy proved more effective at stimulating anti-tumor responses and, importantly, a tumor-specific immune memory in mouse models that kept the animals rejecting tumor cells at least half a year later.
“There is tremendous enthusiasm for using neoantigens in immunotherapy as predicting them in individual tumors becomes more and more reliable. Our materials approach is able to mix and match predicted neoantigens very easily and efficiently in a single scaffold that, as a delivery vehicle, could be plugged into existing pipelines to enable more effective personalized cancer treatments,” said Mooney, who led the study. Mooney also leads the Wyss Institute’s Immunomaterials Platform and is the Robert P. Pinkas Family Professor of Bioengineering at SEAS.
The scientists used their previously developed programmable biomaterial made from tiny mesoporous silica rods (MSRs) that can be injected under the skin, where they spontaneously assemble into a 3-D scaffold that attracts and stimulates DCs. They then coated the MSRs with polyethylenimine (PEI), a polymer used previously to deliver DNA and proteins to cells. PEI has been surmised to have immune-stimulatory effects.
“This allowed us to achieve two things: It enabled ready absorption of multiple peptides regardless of their inherent properties without the need to further modify them; and by being taken up by DCs together with the peptides, PEI enhanced the stimulation of DCs and the ensuing tumor-directed cytotoxic T cell responses in our mouse models,” said first-author Aileen Li, who performed her graduate work with Mooney and now is a postdoctoral fellow at the University of California, San Francisco.
In addition to the PEI coating, the vaccines also contained factors that help them attract DCs and boost immune functions. Comparing them to control vaccines that lacked PEI but had all the other components, the team found them considerably more efficient in activating DC populations, stimulating interactions with T cells in nearby lymph nodes and driving the generation of circulating killer T cells that recognize tumor-specific peptides.
Raising the strategy’s clinical potential, these advances also translated to mouse models with more relevant tumors that the researchers investigated with a collaborating team lead by Kai Wucherpfennig, chair of the Dana-Farber Cancer Institute’s Department of Cancer Immunology and Virology.
First, they designed a vaccine that presented a model peptide of the well-known E7 oncoprotein from human papilloma virus (HPV), which causes cervical and other cancers. Impressively, a single injection of the vaccine led to rapid and complete eradication of HPV tumors in mice, with 80 percent of the animals living longer than 150 days. In comparison, most untreated animals succumbed to the cancer by 30 days, and a control vaccine lacking PEI and a traditionally formulated vaccine had effects only about half as strong. Even six months after the injection, the animals vaccinated with the PEI formulation could still destroy tumor cells, showing that they had formed a robust immunological memory of the tumors.
The team mimicked potential future neoantigen approaches in human patients more closely by carrying out studies in more aggressive and difficult-to-treat tumor models.
“We introduced up to five neoantigens that had been recently identified in mouse melanoma and colorectal tumors into our biomaterial scaffold, and found that a single injection of the vaccines cleared tumor metastases and provided strong immune responses against the tumors that were comparable to multiple injections with existing vaccines,” said Li.
When combined with immune checkpoint therapy, which can broadly invigorate killer T cell activity against tumors, the effects of both the vaccine and the checkpoint therapy were boosted.
Different immune checkpoint therapies are currently performed in the clinic, but their effects in many patients and tumors remain weak. The team thinks that combining them with their biomaterial-supported neoantigen approach could help treat many patients more effectively.
The study was funded by Harvard’s Wyss Institute for Biologically Inspired Engineering, the National Institutes of Health, the Melanoma Research Alliance Foundation, and the National Science Foundation.
This release has been edited for length and clarity. To read the full release, visit the Wyss Institute website.