
Charles Lieber and his colleagues published a paper on neural probes that are less detectable by the human brain and may be more effective in treatment.
Rose Lincoln/Harvard file photo
Sensors go undercover to outsmart the brain
Devices used in mice offer a more accurate way to study the brain, potential treatment for disease, damage, mental illness
Like a well-guarded fortress, the human brain attacks intruders on sight. Foreign objects, including neural probes used to study and treat the brain, do not last long. But now, researchers have designed a probe that looks, acts, and feels so much like a real neuron that the brain cannot identify it as an imposter. According to Charles M. Lieber, this breakthrough “literally blurs the ever-present and clear dissimilarities in properties between man-made and living systems” — in other words, between human and machine.
Lieber, the Joshua and Beth Friedman University Professor at Harvard, and his lab members are authors on a new paper published in Nature Materials that presents a bioinspired design for neural probes. Implanted directly into brain tissue, the probes are designed to survive as long as possible in the organ’s warm, humid, inhospitable environment. Sensors hidden within protective casings send data back to researchers about how and when individual neurons fire and neural circuits communicate. This information could help scientists treat neurological disorders like Parkinson’s, reverse neural decay from Alzheimer’s and aging, and even enhance cognitive capabilities.
But current implants cannot trick the brain — they cause a foreign-body response. Large and stiff compared with real neurons and neural tissue, traditional implants have two major impediments to sustained monitoring. During the initial placement in brain tissue — which usually requires surgery — neurons flee the impacted area. Previous studies have shown that the brain’s immune system senses the foreign object and gets to work, causing inflammation and scar tissue to isolate the device. Even if they can capture signals beyond the scar tissue, rigid probes can shift position and end up replacing one neural signal for another, closer one.
“This will ultimately make the recorded signal unstable,” said first author Xiao Yang, a fourth-year graduate student in the Lieber lab. She moved her cupped hands together, then apart, then together again as she explained how she and her team built a probe that inspires a negligible immune response, records neural signals within a day post-implantation, and may even encourage tissue regeneration.
“The stereotype of the neural probe is that they are giant compared to the neuron targets that they’re interrogating,” she explained. “But in our case, they are essentially the same.” The team’s probe mimics three features that have previously been impossible to achieve in a lab: the shape, size, and flexibility of an actual neuron.
Neuronlike electronics (red) mimic the shape, size, and flexibility of neurons (green), enabling them to act in concert with native brain tissue.
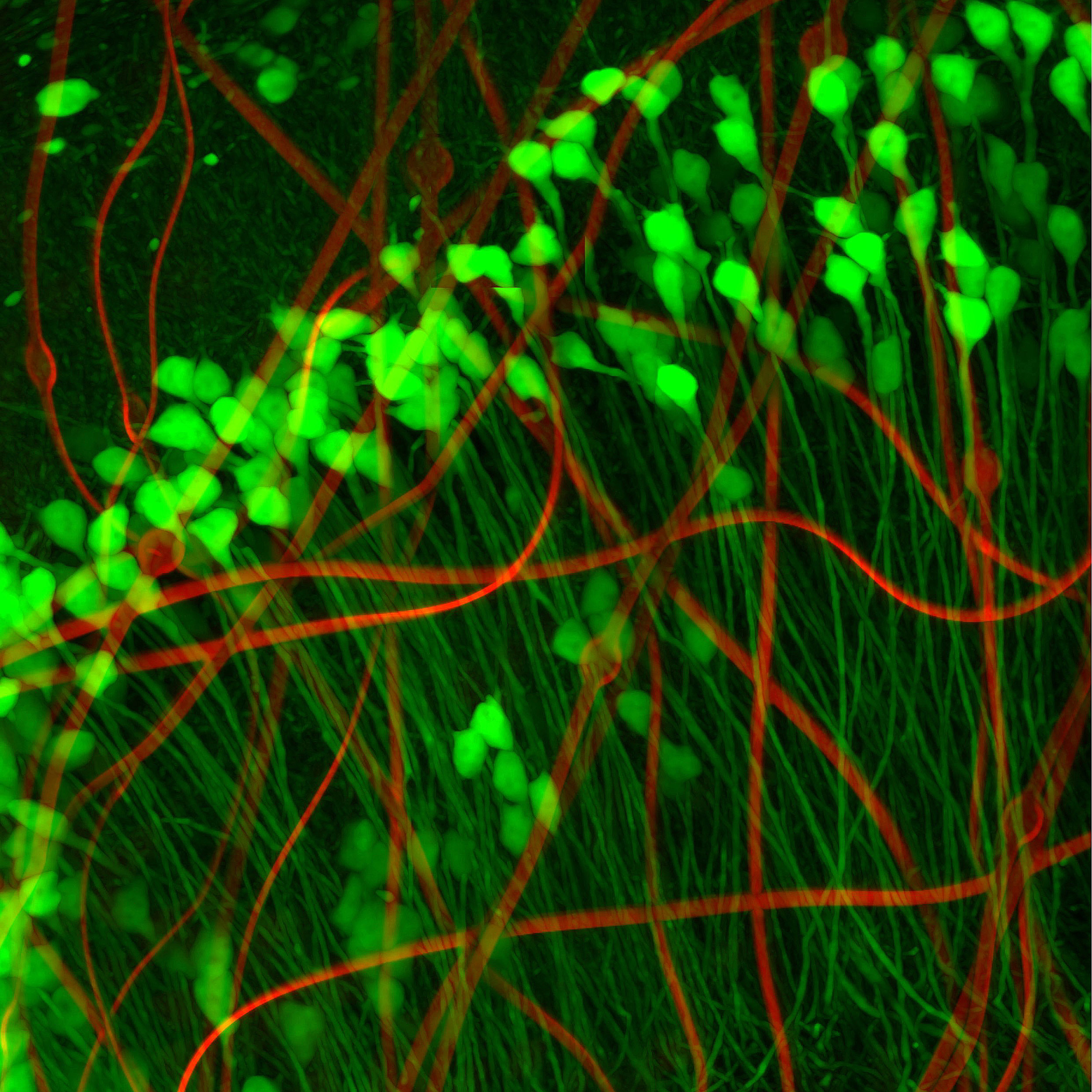
Neurons look a bit like tadpoles, with round “heads” — actually the soma, or cell body — and long, flexible tails. So Yang and her colleagues created a minuscule compartment the same size as the neuron’s soma to house their metal recording electrode. Its wires interconnect — which attaches to input/output pads positioned on the outside of the mouse’s skull to collect and store data from individual sensors — snake through an ultra-flexible polymer “tail,” resembling the neuron’s neurite. According to Yang, their neuron-like electronics (NeuEs) are “five to 20 times more flexible than the most flexible probes reported to date.” The ones they bested were their own mesh electronics, developed last year.
The width of a typical neuron soma is about the same as a very fine strand of hair (20 microns), and the “tail” can be 10 to 20 times finer. Measuring the same or even thinner widths, the neuron-like electronic is the smallest probe yet. To craft their microscopic tools, Yang and her colleagues relied on photolithography, which uses light to transfer a pattern onto material and constructs the probe’s four distinct layers of metal and polymer one at a time.
Once the devices are built, the team uses a syringe to inject 16 of their cell imitators into the hippocampus region — chosen for its central role in learning, memory, and aging — of a mouse brain. There, the NeuEs unfold to create a porous web, imitating the brain’s crisscrossing neural network.
Bigger, less-flexible probes — the next-smallest, created by the same team, are five to 50 times larger than the NeuEs — displace native cells from their territory and can disrupt the neural circuits that researchers are trying to study. Yang’s probes allow cells to integrate fully, and take up less than 1 percent of the volume where they are implanted. Starting from as early as a day to months later, real neurons integrate with the artificial network, forming a harmonious hybrid. This assimilation explains why the team achieved stable data collection even months post-implantation. They did not lose even one neuron signal. Instead, they actually gained some.
Yang called this “an unexpected and exciting result,” explaining that the new signals indicate that newborn neurons may use the artificial neuron-like electronics as a scaffold to reach damaged areas of the brain and help regenerate tissue.
Though further research is needed, the neuron-like electronics could eventually offer a safe, stable alternative to treat neurological diseases, brain damage, and even depression and schizophrenia, where they can provide the added benefit of actively monitoring and modulating regenerated neural networks. Regenerative treatments typically rely on stem cells to help the brain rebuild after damage. But, like larger probes, transplanted stem cells can cause an immune response, which weakens their efficacy. Neuron-like electronics instead recruit endogenous stem cells from the host’s brain and help them migrate to the damaged region. Since they are not perceived as foreign objects, the brain’s immune system lets them work in peace.
Currently, Yang is working in several directions, including designing and fabricating even smaller and more flexible probes and exploring the potential of the NeuEs to serve as an active scaffold for regenerating neural tissue in vivo. With marginal immune response, regenerative properties, and unprecedented stability, the NeuEs not only blur the line between man-made and living systems, they make it near invisible.
Harvard’s Star-Friedman Challenge for Promising Scientific Research provided critical support to the research’s early stages.







