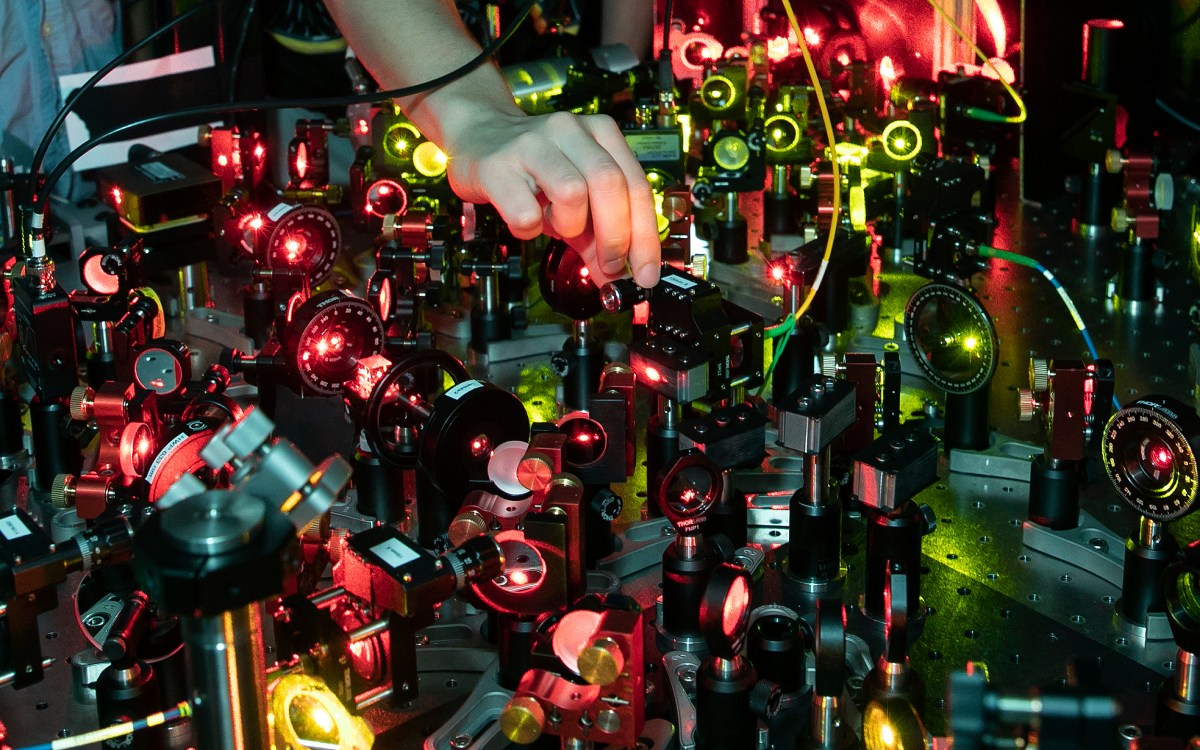
Working with lasers in the Doyle Lab.
Kris Snibbe/Harvard file photo
A cool first for Harvard
By slowing polyatomic molecule, scientists open new paths of quantum study
After firing the lasers and bombarding the ultracold molecule with light, the scientists gathered around the camera to check the results. By seeing how far the molecule expanded they would know almost instantly whether they were on the right track to chart new paths in quantum science by being the first to cool — aka, slow down — a particularly complex, six-atom molecule using nothing but light.
“When we started out on the project we were optimistic but were not sure that we would see something that would show a very dramatic effect,” said Debayan Mitra, a postdoctoral researcher in Harvard’s Doyle Research Group. “We thought that we would need more evidence to prove that we were actually cooling the molecule, but then when we saw the signal, it was like, ‘Yeah, nobody will doubt that.’ It was big and it was right there.”
The study led by Mitra and graduate student Nathaniel B. Vilas is the focus of a new paper published in Science. In it, the group describes using a novel method combining cryogenic technology and direct laser light to cool the nonlinear polyatomic molecule calcium monomethoxide (CaOCH3) to just above absolute zero.
The scientists believe their experiment marks the first time such a large complex molecule has been cooled using laser light, and say it opens new avenues of study in quantum simulation and computation, particle physics, and quantum chemistry.
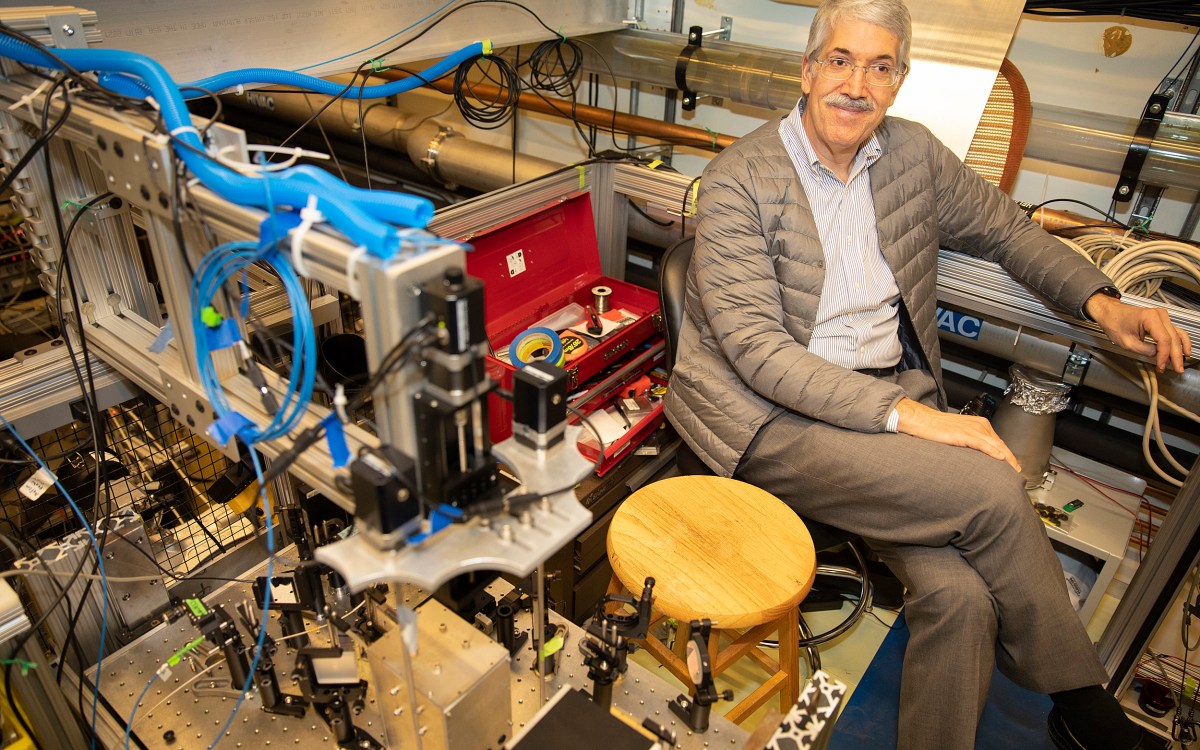
“These kinds of molecules have structure that is ubiquitous in chemical and biological systems,” said John M. Doyle, the Henry B. Silsbee Professor of Physics and senior author on the paper. “Controlling perfectly their quantum states is basic research that could shed light on fundamental quantum processes in these building blocks of nature.”
Inside the lab of John Doyle (pictured), Harvard researchers were the first to cool a polyatomic molecule using light.
Kris Snibbe/Harvard file photo
The use of lasers to control and atoms and molecules — the eventual building blocks of quantum computers — has been practiced since the 1960s and has since revolutionized atomic, molecular, and optical physics.
The technique essentially works by firing a laser at the atoms and molecules, causing them to absorb the photons from the light and recoil in the opposite direction. This eventually slows them down and even stops them in their tracks. When this happens, quantum mechanics becomes the dominant way to describe and study their motions.
“The idea is that on one end of the spectrum there are atoms that have very few quantum states,” Doyle said. Because of this, these atoms are easy to control with light, since they often remain in the same quantum state after absorbing and emitting light, he said. “With molecules, they have motion that does not occur in atoms — vibrations and rotations. When the molecule absorbs and emits light this process can sometimes make the molecule spin around or vibrate internally. When this happens, it is now in a different quantum state and absorbing and emitting light no longer works [to cool it]. We have to ‘calm the molecule down,’ get rid of its extra vibration before it can interact with the light the way we want.”
Scientists — including those from the Doyle Group which is part of the Harvard Department of Physics and a member of the Harvard-MIT Center for Ultracold Atoms — have been able to cool a number of molecules using light, including diatomic and triatomic molecules, which each have two or three atoms.
Polyatomic molecules, on the other hand, are much more complex and have proven much harder to manipulate because of all the vibrations and rotations.
To get around this, the group used a method they pioneered to cool diatomic and triatomic molecules. Researchers set up a sealed cryogenic chamber where they cooled helium to below four Kelvin (nearly 450 degrees below zero Fahrenheit). This chamber essentially acts as a refrigerator, in which the scientists created the molecule CaOCH3. Right off the bat, it began moving at a much slower velocity than it would normally, making it ideal for further cooling.
Next came the lasers. They turned on two beams of light on the molecule, coming from opposing directions. The counterpropagating lasers prompted a reaction known as Sisyphus cooling. The reaction takes its name from the myth of Sisyphus, a Greek king who angered Zeus and was doomed to roll a giant boulder up a hill for eternity, only for it to roll back down when he nears the top.
Essentially the same thing happens here with the molecule, Mitra said. When two identical laser beams are firing in opposite directions, they form a standing wave of light, stronger in some places and less intense in others. This wave forms a metaphorical hill for the molecule.
The molecule “starts at the bottom of a hill formed by the counter-propagating laser beams, and it starts climbing that hill just because it has some kinetic energy in it and as it climbs that hill, slowly, the kinetic energy that was its velocity gets converted into potential energy and it slows down and slows down and slows down until it gets to the top of the hill where it’s the slowest,” Mitra said.
At that point, the molecule moves closer to a region where the light intensity is high and the molecule is more likely absorb a photon that causes it to roll back down to the opposite side. “All [it] can do is keep doing this again and again and again,” Mitra said.
By looking at images from cameras placed outside the sealed chamber, the scientists inspect how much a cloud of these molecules expands as it travels through the system. The narrower the cloud, the less kinetic energy it has — and therefore the colder it is.
Analyzing the data further, the researchers saw just how cold. They took it from 22 millikelvin to about 1 millikelvin — just a few thousandths of a decimal point above absolute zero.
The paper lays out ways to get the molecule even colder, and discusses some of the pathways that opens in a range of physical and chemical research frontiers. The scientists said the study is proof of concept that their method could be used to cool other carefully chosen complex molecules to advance quantum science.
“What we did here is sort of extending the state of the art,” Mitra said. “It’s always been debated whether we would ever have technology that will be good enough to control complex molecules at the quantum level. This particular experiment is just a stepping stone.”
This research was supported with funding from the National Science Foundation.