The 40,000-square-foot facility in Watertown will house the center for advanced biological innovation and manufacturing.
Images courtesy of Alexandria Real Estate Equities, Inc.
Big step forward for planned center to boost cell- and gene-therapy advances
Partnership signs Watertown lease on path to opening facility next year
An innovative public-private partnership took a big step in its plan to open a center next year that aims at boosting advances in cell and gene therapy in the region, signing a 10-year lease for a 40,000-square-foot facility in Watertown.
Project participants refer to the facility, which has not been formally named, as the center for advanced biological innovation and manufacturing (CABIM). The goal is to increase availability of materials like genetically altered cells that are essential to advancing discoveries from the lab to clinics for use in treating patients.
“There has been great progress in developing pharmaceuticals — small-molecule drugs — to treat a wide range of diseases,” said Harvard Provost Alan Garber, who has led the effort. “But many conditions resist treatment with conventional pharmaceuticals. Cell-based therapies offer biological approaches that are complementary to and sometimes far more effective than chemistry-based treatments.”
Scientists say that a bottleneck in manufacturing such biological materials is slowing the development of cutting-edge advances in gene therapy, stem cell science, regenerative medicine, CRISPR/Cas9 gene editing, and cancer immunotherapy. An array of treatments based on those and similar technologies — such as those involving RNA, peptides, and oligonucleotides — are in development, in clinical trials, and in some cases already in the clinic.
“This facility will help turn scientific findings into approved therapies by making these resources available to early-stage companies and labs.”
Alan Garber, Harvard provost
The center, whose creation was announced in late 2019, is led by institutions from both academia and industry. It will contain both manufacturing and innovation space to boost the supply of materials for late-stage research and early clinical trials and provide space to develop ideas that have left the lab but are not yet ripe for corporate investment. It will also emphasize training in the operation of advanced equipment used in cell manufacturing as a way to increase the pool of workers with such critical skills in the region.
“The promise of cell-based therapies has been proven,” Garber said, pointing to recent gene-therapy trials to treat sickle cell anemia, which showed significant improvement. He also cited stem-cell-based work to treat diabetes by implanting insulin-producing beta cells, developed in the Harvard lab of Xander University Professor Douglas Melton.
“The development of tools like CRISPR and progress in stem-cell science are among the advances that have given us hope that we will soon be able to treat cancer, immunological diseases, neurological conditions, and many other inherited conditions far better,” Garber said. “This facility will help turn scientific findings into approved therapies by making these resources available to early-stage companies and labs.”
Spearheaded by Harvard University and the Massachusetts Institute of Technology, the center is led by a partnership whose other members are Fujifilm Diosynth Biotechnologies, Cytiva, and Alexandria Real Estate Equities. Additional partners include Beth Israel Deaconess Medical Center, Boston Children’s Hospital, Brigham and Women’s Hospital, the Dana-Farber Cancer Institute, Massachusetts General Hospital, and MilliporeSigma.
“Our goal is to do our part to help keep the life sciences community in Boston vibrant for decades to come,” Garber said. “By investing in this facility, we hope to nurture continued innovation in one of the most promising areas of the life sciences.”
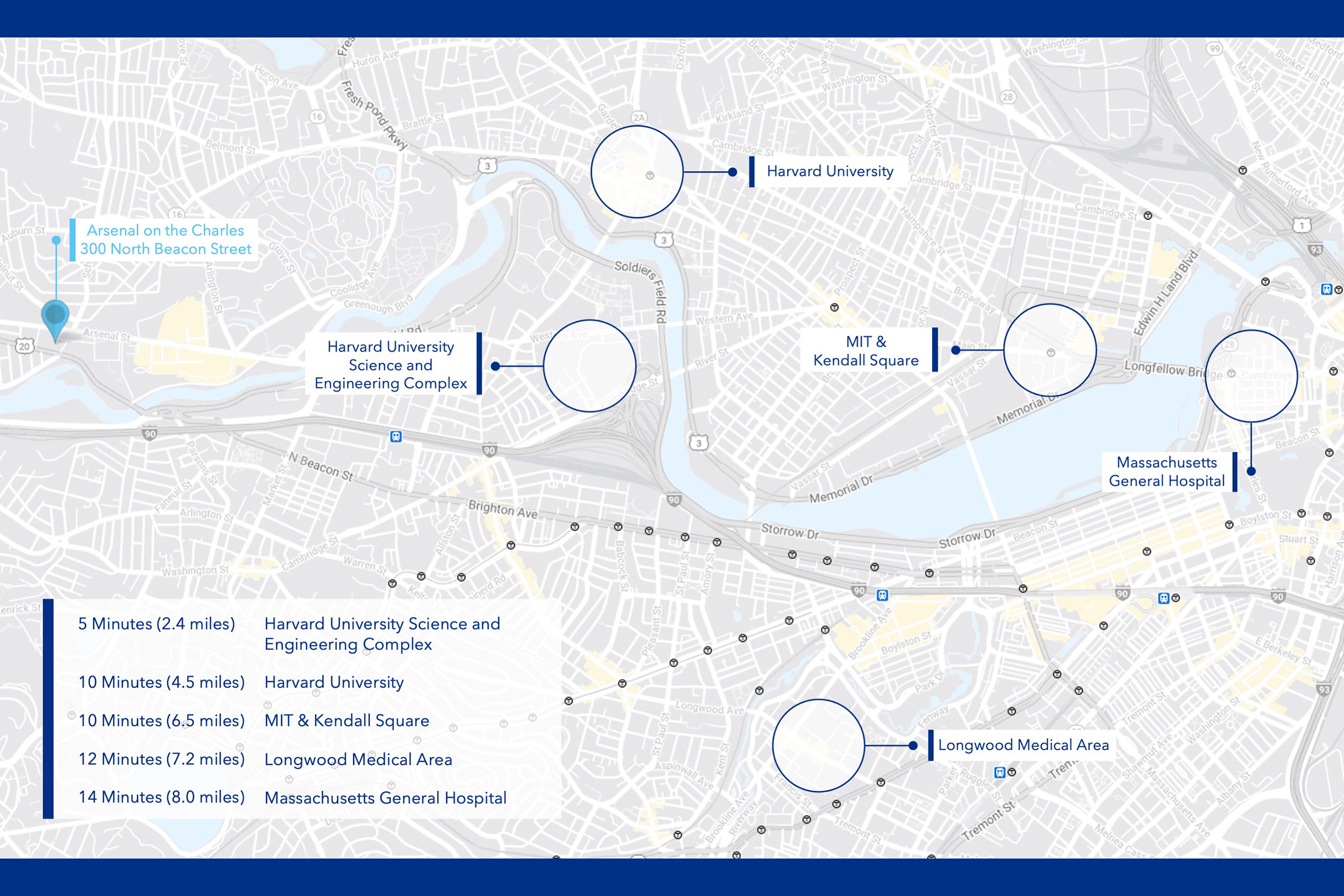
Organized as a private nonprofit, the new facility will have a staff of about 40 researchers experienced in advanced biological manufacturing techniques. It will be located at 300 North Beacon St. in Watertown, close to a number of biotech startups in the Watertown Life Science Cluster. The location also provides easy access to labs at Harvard — including its new Science and Engineering Complex — and MIT as well as to other partners in Greater Boston.
The center’s initial development and operation is being supported by $76 million raised by its partners. It grew out of a years-long strategic planning process done in partnership with state officials. Conducted by the Massachusetts Life Sciences Strategies Group, its aim was to identify areas that might be important to the region’s future economic growth, with an emphasis on maintaining Greater Boston’s leadership in life sciences and biotechnology. Garber and Vice Provost for Research Richard McCullough were among the effort’s leaders, and they are continuing those roles in the development of the new center. The strategic group focused on finding practical steps that would boost promising, cutting-edge technology likely to become mainstream economic drivers in the years to come.
They settled on cell and gene therapy, a budding field that has seen near-miraculous results — such as the dramatic clearing of stubborn cancers through the use of cancer immunotherapy or the treatment of genetic-based ailments like sickle cell disease by altering their roots in DNA, an approach that promises not just easing of symptoms but, potentially, a cure — something that not long ago seemed unthinkable.
“They’re getting remarkable success in a variety of clinical trials and could even lead to a possible cure,” McCullough said, citing dramatic results treating leukemias and other blood cancers. “One of the major goals of the CABIM facility is to accelerate innovation and patient treatments using biological therapeutics.”
Those successes have not come without setbacks, however. Some approaches work well with some individuals but have little effect on others; others spark severe reactions or have other drawbacks. A supply of engineered cells, viral vectors and other biological materials is needed by scientists working to understand those reactions and develop new approaches, but work can be stalled for months as researchers wait for commercial cell manufacturing facilities to fill orders.
“Time will tell,” McCullough said, “but it really does feel like the beginning of something quite significant.”